
מזמינים אתכם לסדנה לתיקון פגמים בסחוס ופגמים אוסטאוכונדרליים של הברך
רישום לסדנה שתתקיים
בתאריך 26.3
בין השעות 12:00-13:00 רישום לסדנה שתתקיים
בתאריך 27.3
בין השעות 8:30-10:00
בתאריכים 26-27.3 יתקיים הכינוס ה-44 של האיגוד הישראלי לאורתופדיה 2025, במסגרתו נקיים 2 סדנאות אודות המשתל CARTIHEAL AGILI-C.
בסדנא נלמד אודות האלמנטים הביולוגים הייחודיים של המשתל ונקיים הדרכה על טכניקת השתלת המוצר על גם מודל דמוי עצם.
About
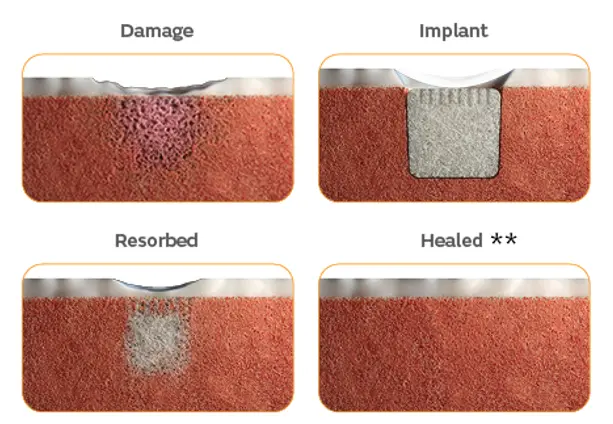
An off-the-shelf solution with a single-stage procedure for knee cartilage and osteochondral defect repair, comprised of a proprietary implant biomaterial that is porous, biocompatible and biodegradable.
Derived from a naturally occurring calcium carbonate known as aragonite, the CARTIHEAL AGILI-C Implant is a porous, biodegradable and biphasic biomaterial scaffold for cartilage regeneration.
Shown to deliver clinically meaningful improvements in pain, function and quality of life, the CARTIHEAL AGILI-C Implant is the only device approved for the treatment of knee cartilage and osteochondral defects in patients with or without mild to moderate osteoarthritis (KL 0-3).
Effective: Twice the pain reduction.
Versatile: Treat small and large lesions, with or without the presence of osteoarthritis(OA)
Convenient: Implanted using a single, simple surgical procedure, with no need for donor matching or cell harvesting
Features
Straightforward surgical procedure
The CARTIHEAL AGILI-C Implant is an off-the-shelf solution, implanted using a single-stage surgical procedure.

Improved outcomes
In pivotal IDE study results, the CARTIHEAL AGILI-C Implant delivered clinically meaningful improvementsin pain, function and patient quality of life when compared to pre-operative scores.
Absorbable implant
Porous, biocompatible and biodegradable implant material that is absorbed.

Superior to the current standard of care
Clinically superior in the areas of KOOS and IKDC scores, MRI defect fill and response rate, as demonstratedin a large, multi-center, randomized and controlled clinical trial (26 sites, 251 patients).